Ballad Health urges continued mask wearing, those recovered to donate plasma
Published 4:32 pm Wednesday, August 26, 2020
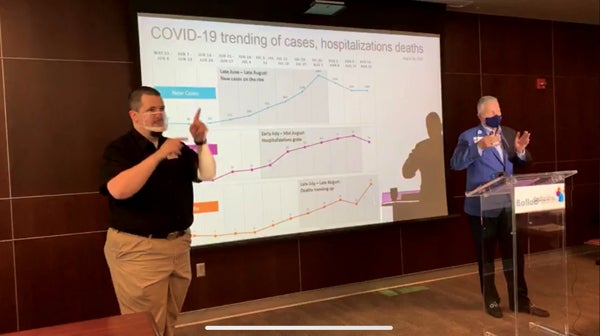
- Brittnee Nave/Star Photo Ballad officials provided the latest updates during their weekly media briefing on Wednesday.
|
Getting your Trinity Audio player ready...
|
Ballad Health officials provided updates on testing, convalescent plasma and the latest COVID-19 trends during their weekly media briefing on Wednesday.
During the briefing, Ballad announced that they will be expanding their in-house testing footprint for both hospital and ambulatory sites. To do this, they are working with Cepheid, a diagnostics company. They are working to be able to offer a test that identifies Flu types A and B, RSV and COVID-19.
The health organization will also be expanding the number of testing sites to test for the virus.
“This will help us prepare for flu season and continue caring for our patients during this pandemic,” said Jamie Swift, Chief Infection Prevention Officer.
In-house testing is currently taking about 24-36 hours.
Tests sent to outside labs are also having better turnaround times now at three to five days.
“Turnaround time is much faster,” said Eric Deaton, Chief Operating Officer. “It’s much, much better.”
As Deaton showed the latest models of the virus, he continued to stress the importance of wearing a mask and the importance of mask mandates.
“We do believe that the mask mandates are working,” he said. “These have been implemented across the region and we are coming up to the date when many of these expire; we would like to see them extended because it’s working.”
In addition to wearing masks, social distancing and continued hand hygiene is also being urged to prevent the spread.
According to Deaton, the Emergency Operations Center, which was set up 180 days ago, is continuing to work on a day to day basis.
Lisa Smithgall, Chief Nursing Executive, took the floor after Deaton and spoke on convalescent plasma.
She recalled that in April, the health organization announced their participation in a study with the Mayo Clinic. Since then, 400 units of convalescent plasma has been collected and has helped many patients. On Monday the FDA issued an emergency use authorization for use of the convalescent plasma, based on scientific evidence that it may be effective in treating the virus.
The plasma is used on patients with moderate symptoms so they do not develop more life-threatening complications.
“We have patients who have told us that the plasma treatment saved their life,” Smithgall said.
Those who have recovered from the virus are asked to donate, which can be done by contacting the Marsh Regional Blood Center at 423-230-5640. Free antibody testing and blood donations are also available by contacting them as well.
There are currently 92 in-house patients, with 20 in the ICU and 16 on ventilators. There are also 18 PUIs (patients under investigation).
For the latest Ballad Health updates, you can go to www.balladhealth.org.





